Reversible Polymerization
Most, if not all, polycondensation reactions are reversible chemical reactions in which the concentrations of monomers, polymer and byproduct reach equilibrium values as the reaction proceeds. A classic example is esterification of a diol with a diacid such as ethylene glycol and terephthalic acid which yields polyethylene terephthalate (PET), commonly known as "polyester". In a closed system, the concentration of water and polyester will build up with increasing conversion until the rate of the reverse reaction (depolymerization) becomes equal to the rate of polymerization:1
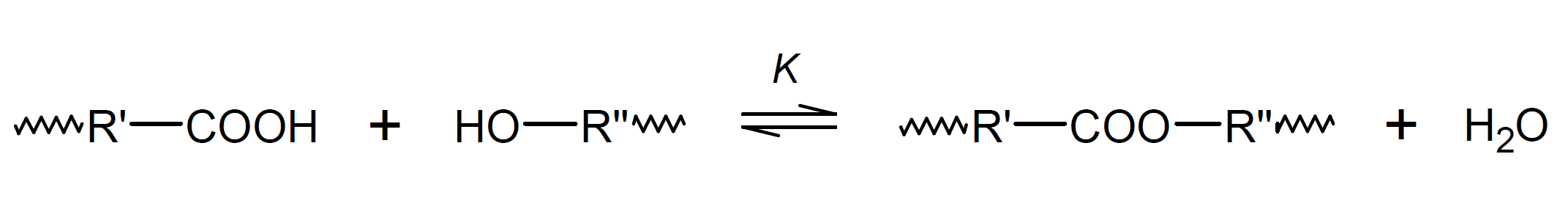
Assuming that all esterification steps - that is the formation of dimers, trimers, and so forth - have the same equilibrium constant (K), the average degree of polymerization and the concentration of residual reactive groups can be easily calculated from the equilibrium constant for polymerization. For a stoichiometric ratio of acid groups [COOH] and alcohol groups [OH], the equilibrium constant for polyesterification is given by:9
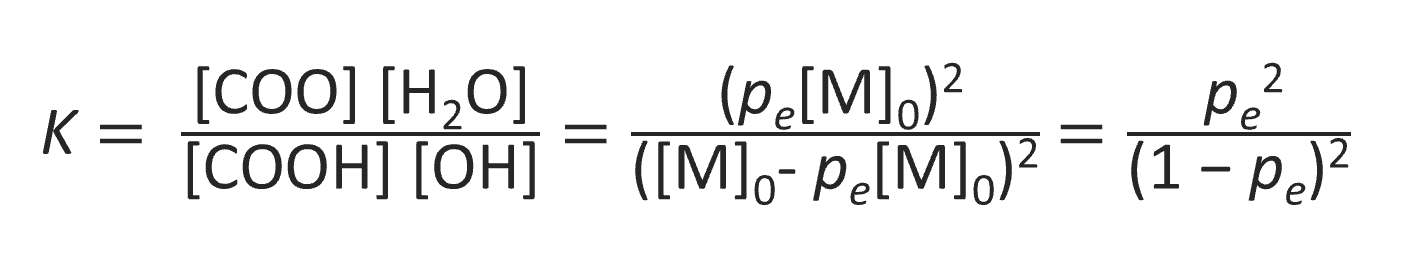
where [M]0 is the initial concentration of hydroxyl and carboxyl groups and pe is the extent of reaction at equilibrium so that pe[M]0 is equal to the concentration of water at equilibrium and (1 - pe) [M]0 to that of hydroxyl and carboxyl groups, respectively. Solving the equation for the extent of reaction, pe, one obtains
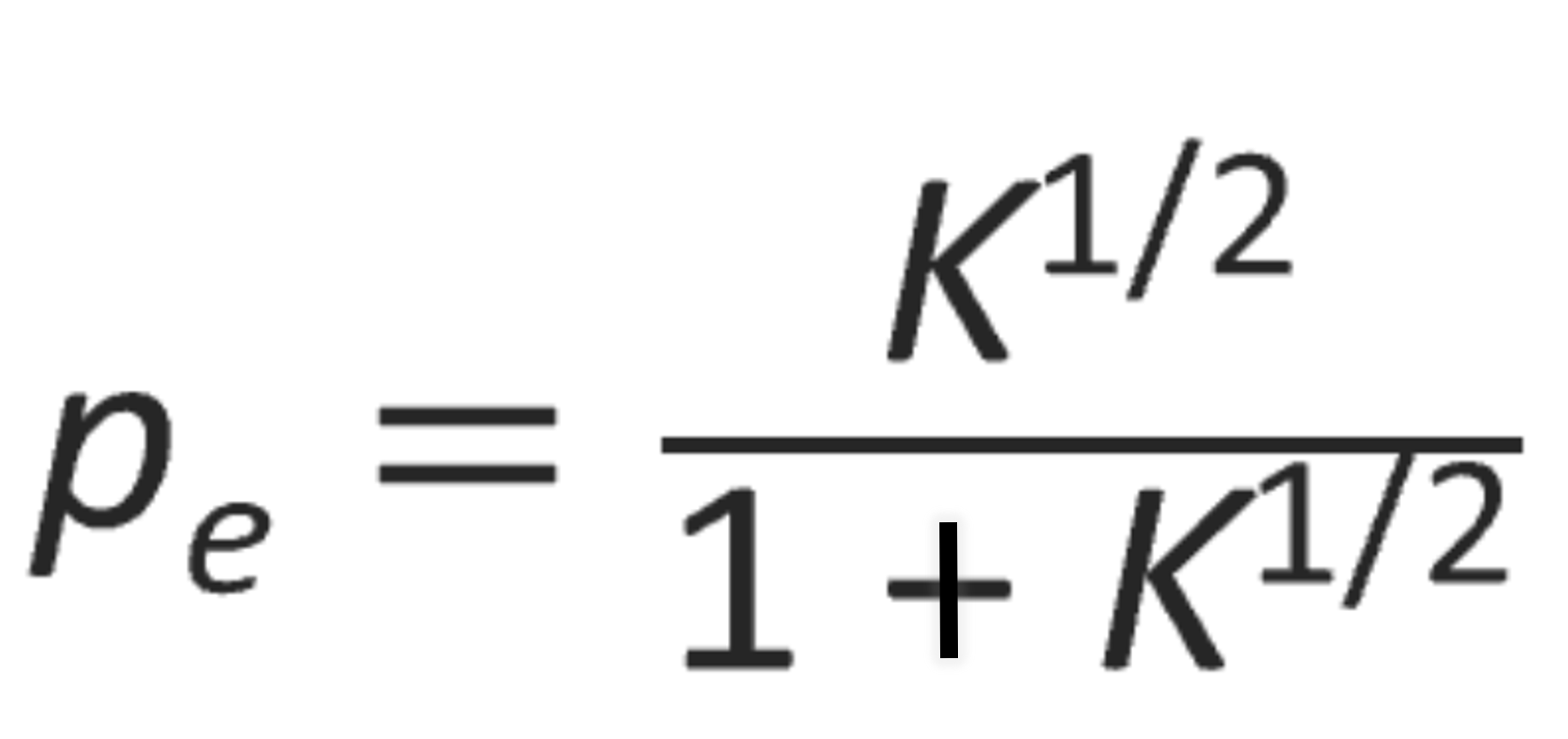
and for the number average degree of polymerization Xn:
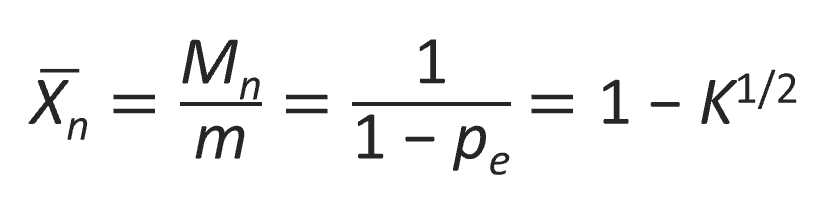
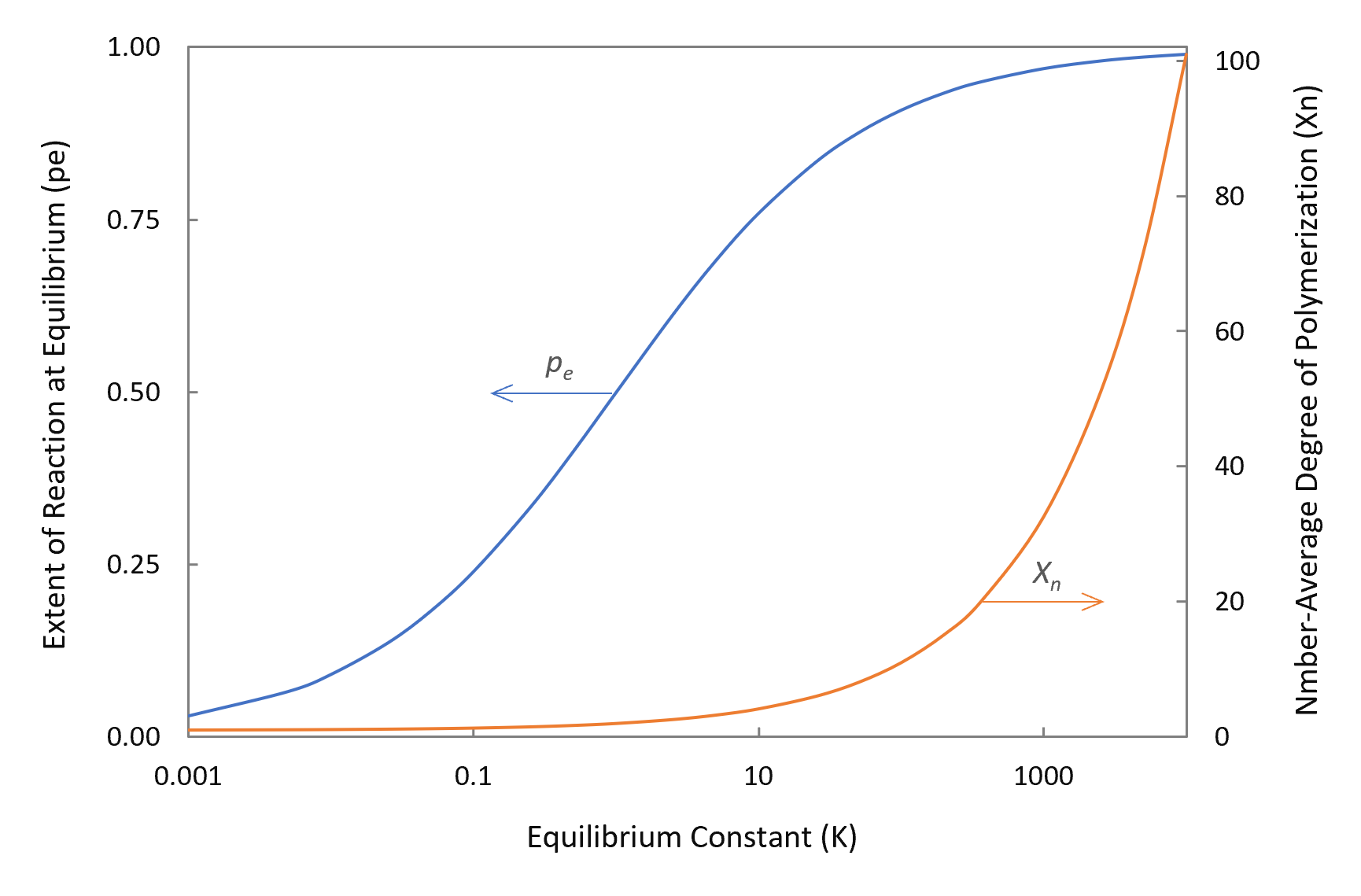
The figure below shows pe and Xn as a function of the equilibrium constant K. The curves clearly demonstrate that in closed systems high conversions and high molecular weights (MW) can only be obtained for very large equilibrium constants. For example, a degree of polymerization of 100 requires an equilibrium constant of about 104. However, K values of polyesterification and transesterification reactions are typically in the range 0.1 - 0.22-5,10 whereas the equilibrium constants of polyamidation reactions
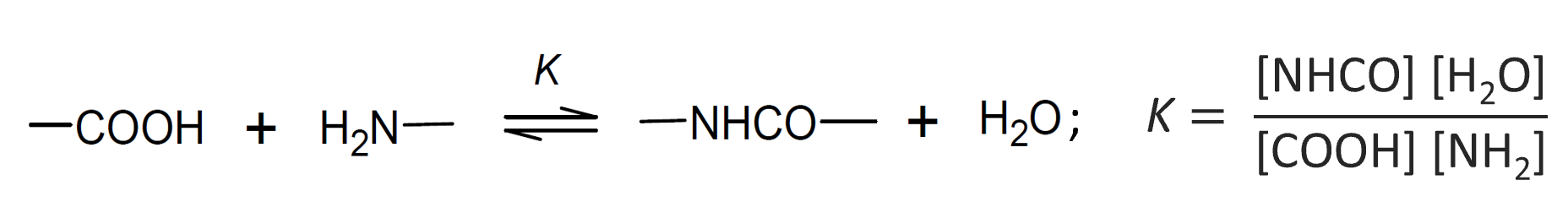
are typically in the range 10 - 1000.6-8 Although the equilibrium constant of amidation is much larger than that of esterification, it is still too small to obtain high-molecular-weight polyamides without removing water.
The majority of step-growth polymerizations are carried out in open systems to remove the low molecular byproducts such as water (esterification, amidation), hydrochloric acid (Schotten-Baumann) or methanol (transesterification) which drives the equilibrium toward the desired high MW products. This is typically done by increasing the temperature, reducing the pressure and purging with inert gas. The continuous removal of the low MW byproducts, however, can be difficult in mass polymerization at high conversions due to the high viscosity of the high MW polymers and reduced mobility of the molecules. For this resason, polymerization in a solvent that facilitates the removal of the byproducts is sometimes preferred.
As has been shown above, the ultimate average degree of polymerization depends on the concentration of residual byproduct (H2O) remaining in the polymer resin. To calculate the ultimate pe and Xn as a function of water content one has to rewrite the reaction quotient at equilibrium in terms of water concentration:9
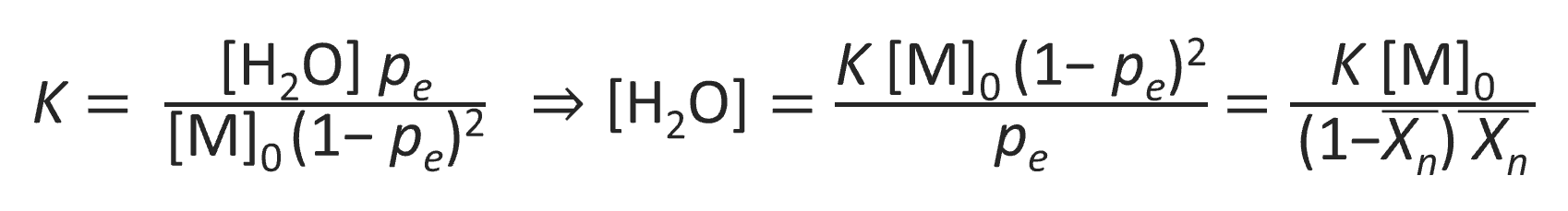
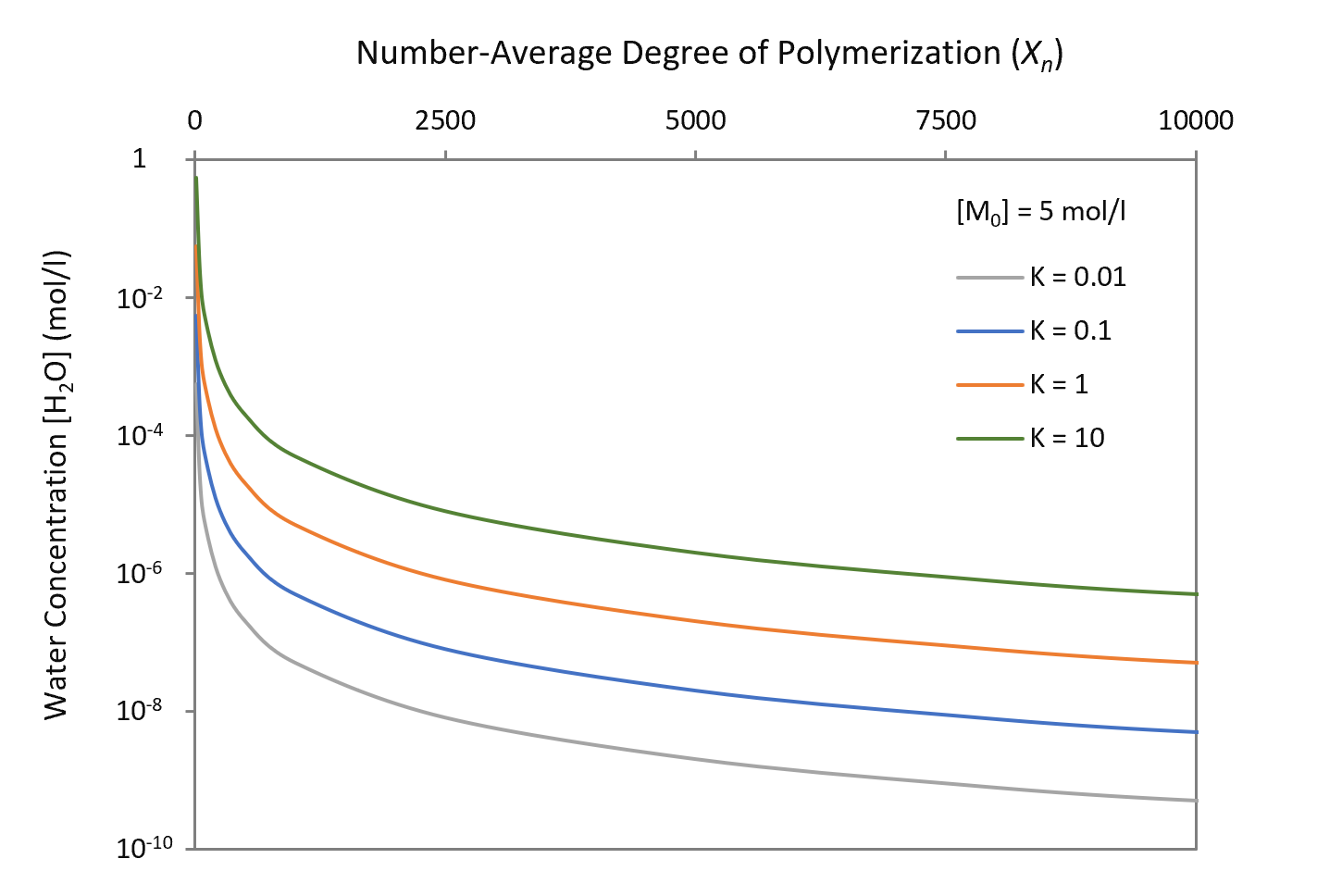
The effect of residual water on the number average degree of polymerization, Xn, for various equilibrium constants (polymers) is shown in the figure above. The curves indicate that the water concentration [H2O] has to be kept extremely low to achieve high molecular weight polymer end products. The required water concentration to achieve a certain degree of polymerization increases with both an increasing equilibrium constant K and an initial concentration of reactants [M]0.
Notes & References
In the case of an esterification, the reverse reaction is also referred to as hydrolysis or glycolysis.
P.J. Darda, K. Hogendoorn, T Molenkamp, & G Versteeg, Macromol. Symp., 206, 275-289 (2004)
C. M. Fontana, J. Poly. Sci.: Part A-1, Vol. 6, pp. 2343 - 2358 (1968)
G. Challa, Macromol. Chem., Vol. 38, pp. 123-137 (1960)
J. Scheirs & T.E. Long, Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, Wiley & Sons (2003)
W. Zheng, K.B. McAuley, E.K. Marchildon & K.Z. Yao, Ind. Eng. Chem. Res., 44, 2675 (2005)
B.L. Deopura, R. Alagirusamy, M. Joshio & B. Gupta, Polyesters & Polyamides, CRC Press 2008
M.V. Tirrell, G.H. Pearson, R.A. WEiss, R.L. Laurence, Poly, Engin. & Sci., 15 (5), 386-393 (1975)
The constant ratio of concentrations of products and reactants is an example of the mass action law. It states if a system is at equilibrium at a given temperature, then the following ratio is a constant:
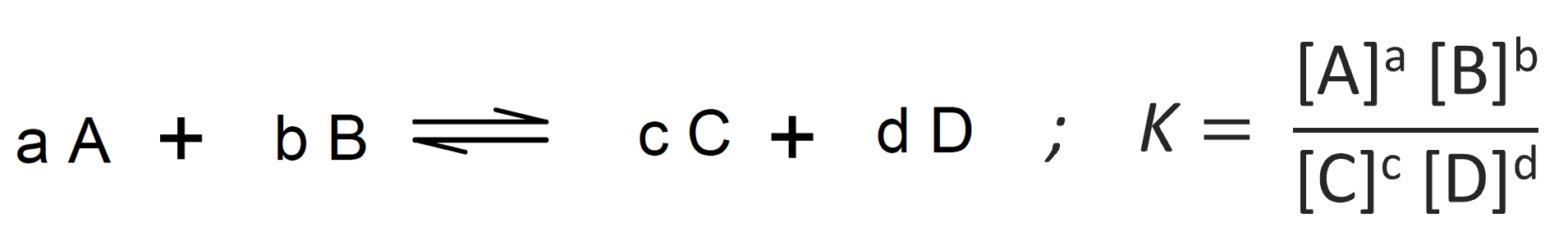
If the system is not at its equilibrium, the ratio is often called the reaction quotient.
Polycondensation equilibrium constants have been measured by several researchers. Fontana3 found that the polycondensation equilibrium constant of PET has a value close to 0.5, being independent of temperature and degree of polymerization and that the normal Flory-Schulz distribution applies to PET, whereas Darda et al.2,4 reported that the equilibrium constant of PBT and PET decreases with increasing degree of polymerization (Xn) at low conversions but is nearly constant above about Xn = 35 (PBT) and 30 (PET), respectively.