Branching in Polymerization
Branching can be encountered in many natural and synthetic polymers and has been observed and investigated almost since the time when linear polymers were synthesized for the very first time.1 One of the oldest theories that describes branching without gelation from a physical point of view was developed by Paul Flory and Walter Stockmayer in the 1940th.2,3 They used a statistical approach and assumed that all molecules including the branched polymers have equal reactivity and that no intramolecular reactions take place. These assumption greatly simplify the calculations but lead to errors in the prediction which Flory assumed to be relatively small in comparison 'to the vast differences between the branched polymer distributiion and that usually prevailing in linear polymers'.5
Over the years, many other theories have been developed that describe random branching in various types of polymers. Some of these theories give more accurate predictions, but the majority of these models are much more complicated and thus, in practice, are less often used.
The molecular weight distribution of a branched condensation or addition polymer can be easily calculated if we assume that each functional group has an equal chance of reacting with other groups regardless of the size of the oligomers. With this assumption, Flory was able to estimate the weight fraction distribution for polymers as a function of degree of polymerization.4,5 He first considered multifunctional monomers of the type ABf-1, that is, monomers with a single functionality of type A and f-1 functional groups of type B, where f is the total number of functionalities per molecule. A may react only with B, whereas reactions between like functional groups are forbidden. For difunctional monomers, f = 2, the addition or condensation of two monomers leads to linear chains, whereas f > 2 leads to randomly branched macromolecules as depicted below for f = 3.
Randomly Branched Molecule
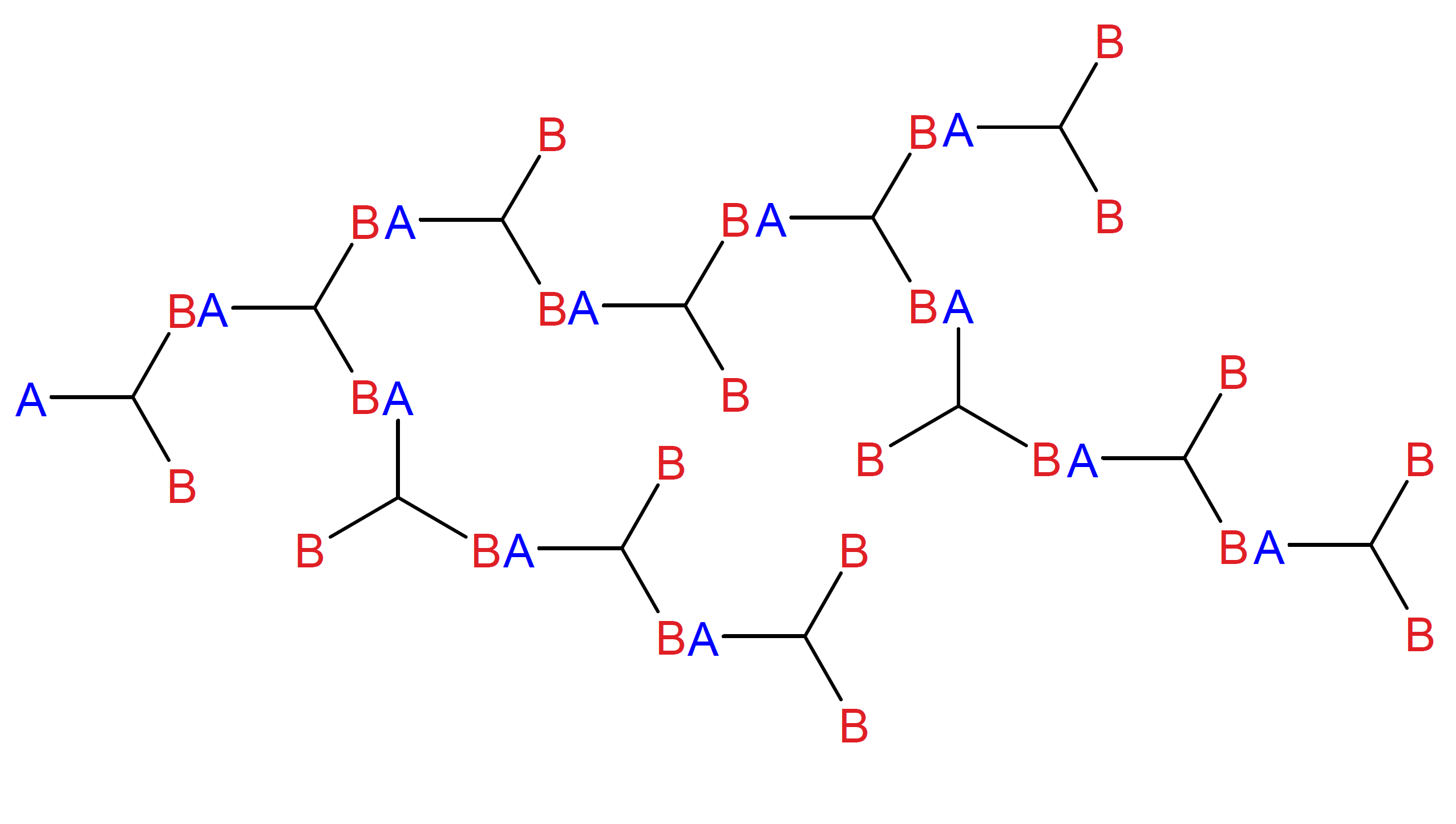
Flory assumed that both monomers and their functional groups are equally reactive, and that a reacted group does not affect the reactivity of unreacted groups of the same unit.7 If p is the fraction of reacted B groups then the fraction of reacted A groups is (f - 1) p because the total number of B groups in ABf-1 is f - 1 times greater and thus the fraction of A groups is consumed f - 1 times faster. The number of unreacted A groups, NA,0 [1 - (f - 1) p], must be equal to the number of molecules Ntot(p) because there is one unreacted A group in every molecule regardless of its size (see figure above). The average number of repeat units per molecule of such a polymerization is equal to NA,0 / Ntot(p), thus
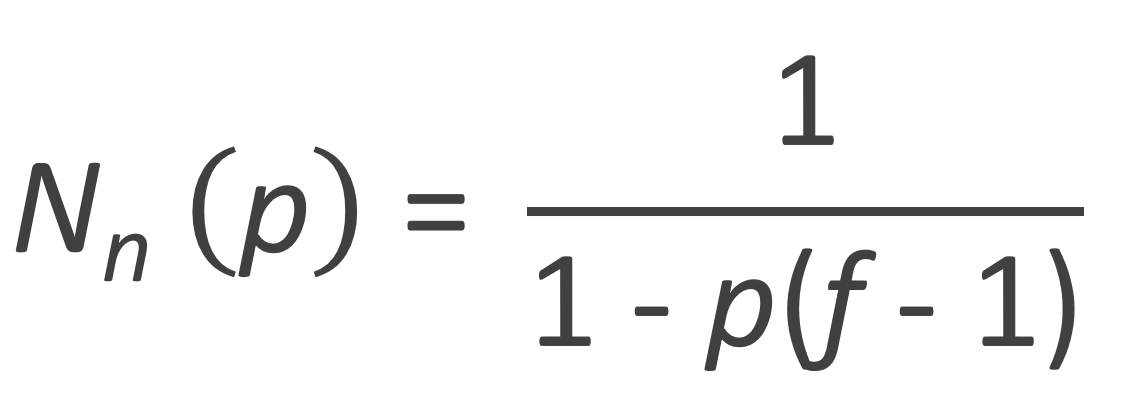
This number diverges at pc = 1 / (f - 1) which is Flory's predicted gel point. In the case of ABf-1 monomers, this is the point at which all A functionalities have reacted, i.e., (f - 1) pc = 1, Thus the monomers form one gigantic branched macromolecule.
The probability that an unreacted A functionality is part of a branched N-mer is proportional to the probability that N - 1 B functionalities have reacted, pN - 1, because there are exactly N - 1 B functionalities in an N-mer. The probability that the remaining N (f - 2) + 1 B groups have not reacted is (1 - p)N(f - 2) + 1. Thus the probability that an A group is attached to an N-mer regardless of its structural configuration is given by5
![]()
where cN is the number of distinguishable ways of arranging N trifunctional monomers into an N-mer. According to Flory, cN can be calculated as5
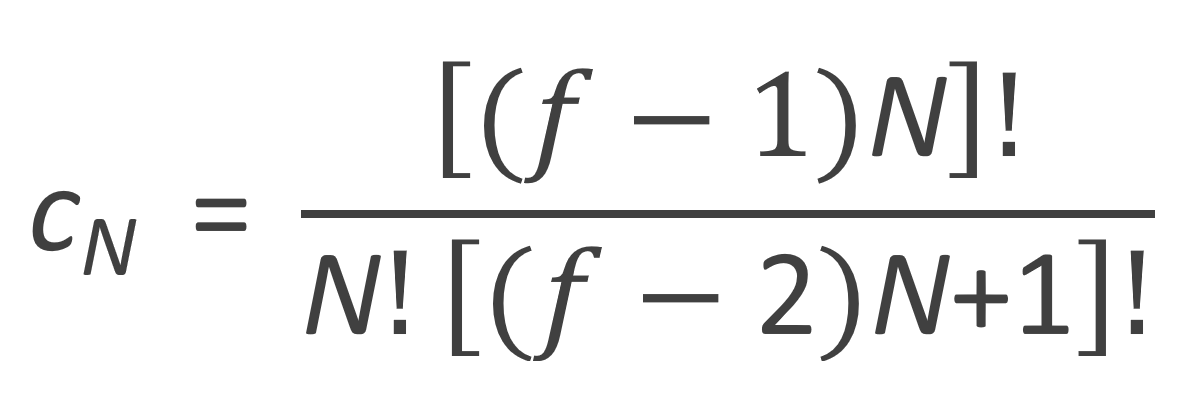
This quantity is called the degeneracy because each of these configurations occurs with the same probability. Using this degeneracy, nN(p) can be written as
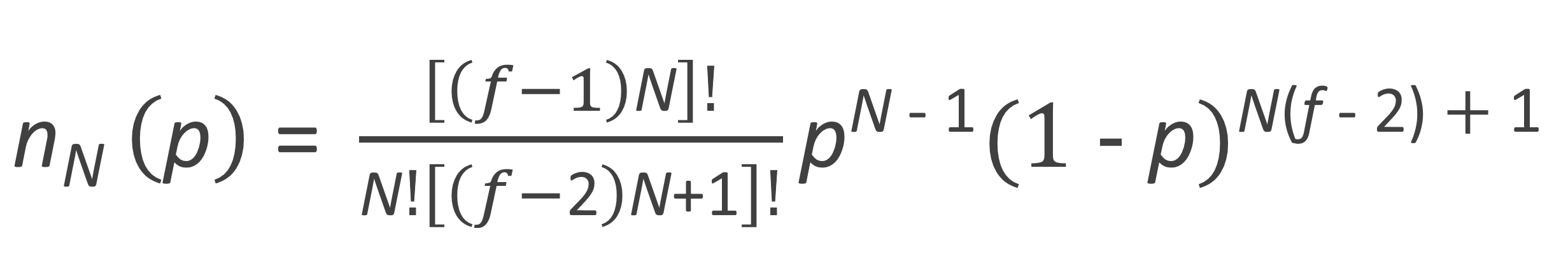
In the absence of intramolecular condensation or additon, each N-mer bears a single unreacted A group. Then nN is also the number fraction of N-mers since the total number of unreacted A's is equal to the total number of molecules.
To calculate the different moments, the number fraction distribution is rewritten as
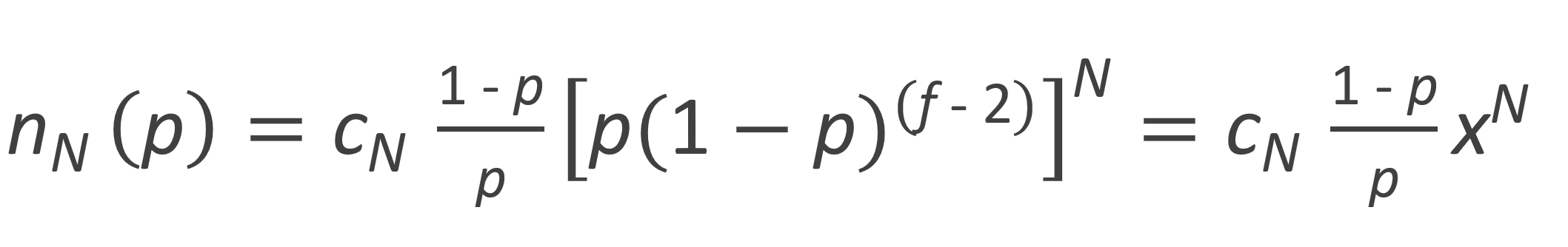
Then the k-th moment of the number fraction distribution can be evaluated from
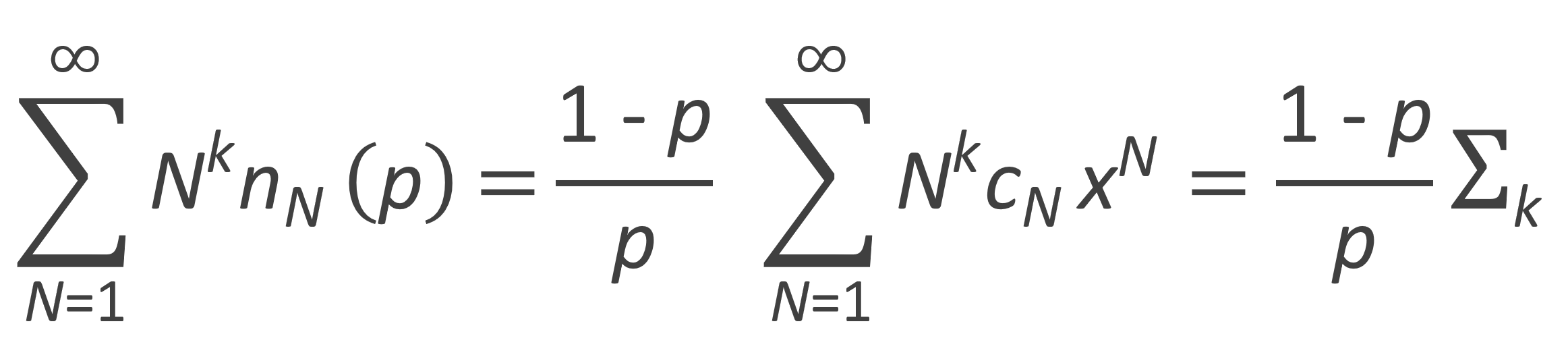
The zeroth, first and second moment of the number fraction distribution are listed in the table below. Flory calculated these moments with following recurrence relation:
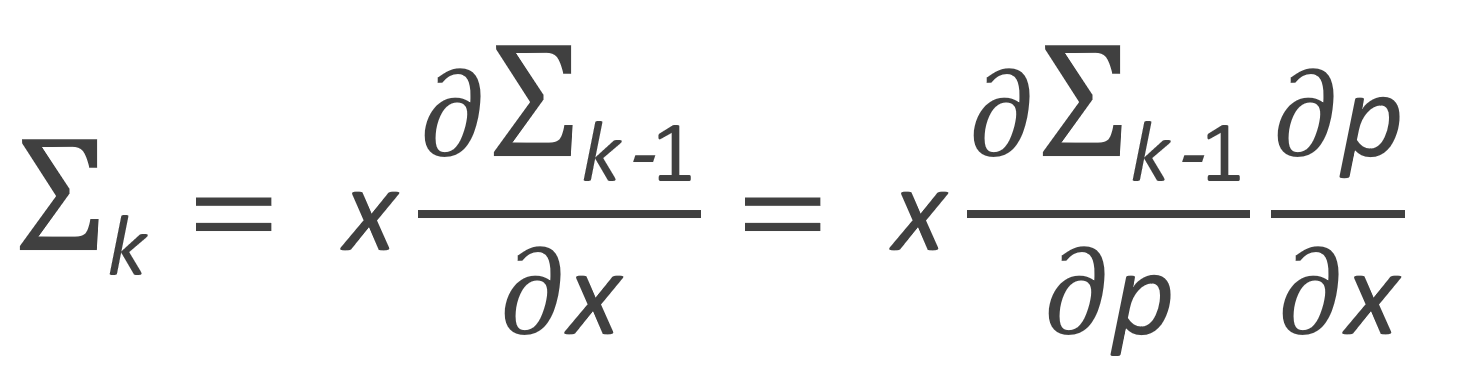
| Moments | 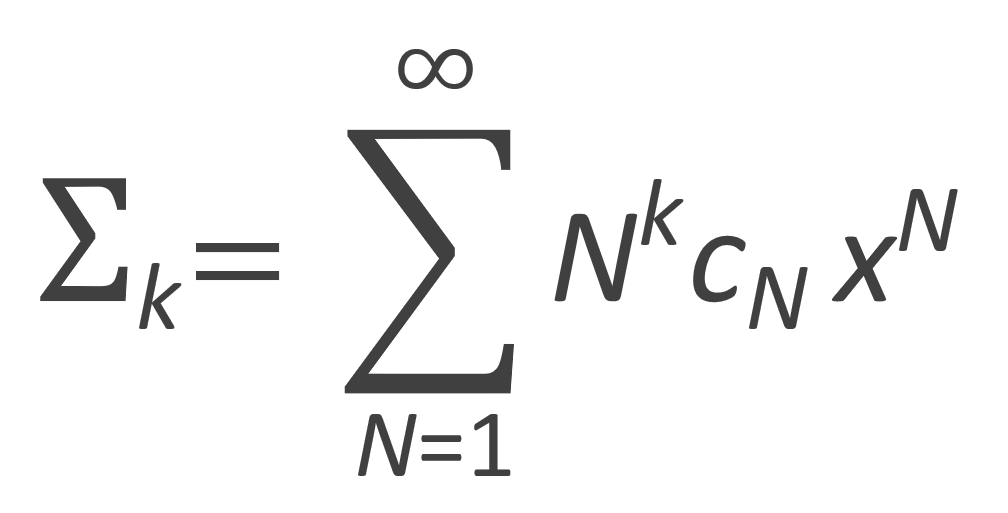 |
| Zeroth Moment | 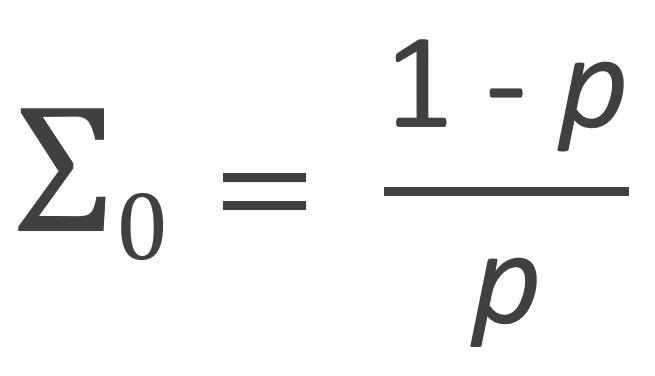 |
| First Moment | 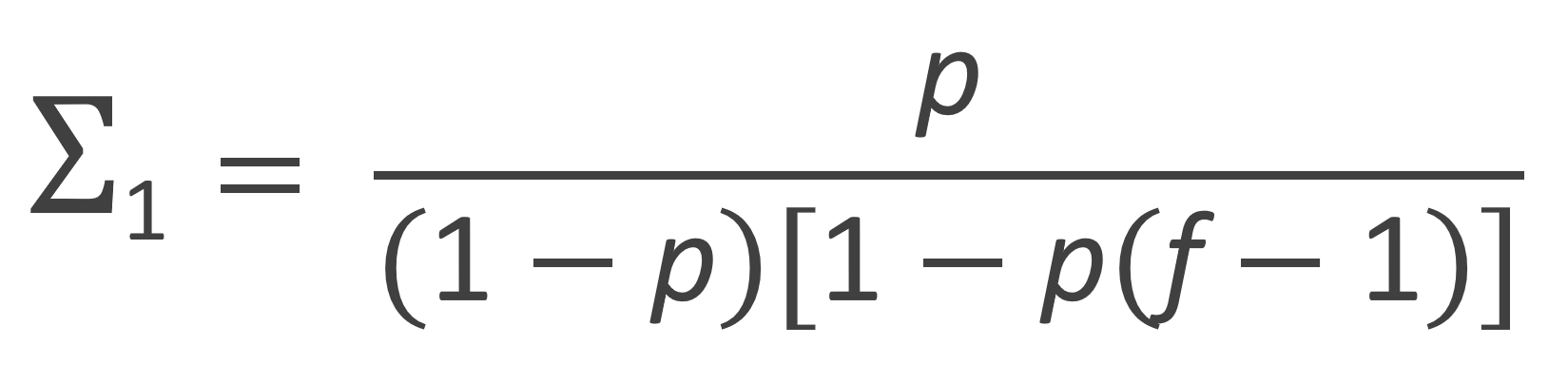 |
| Second Moment | 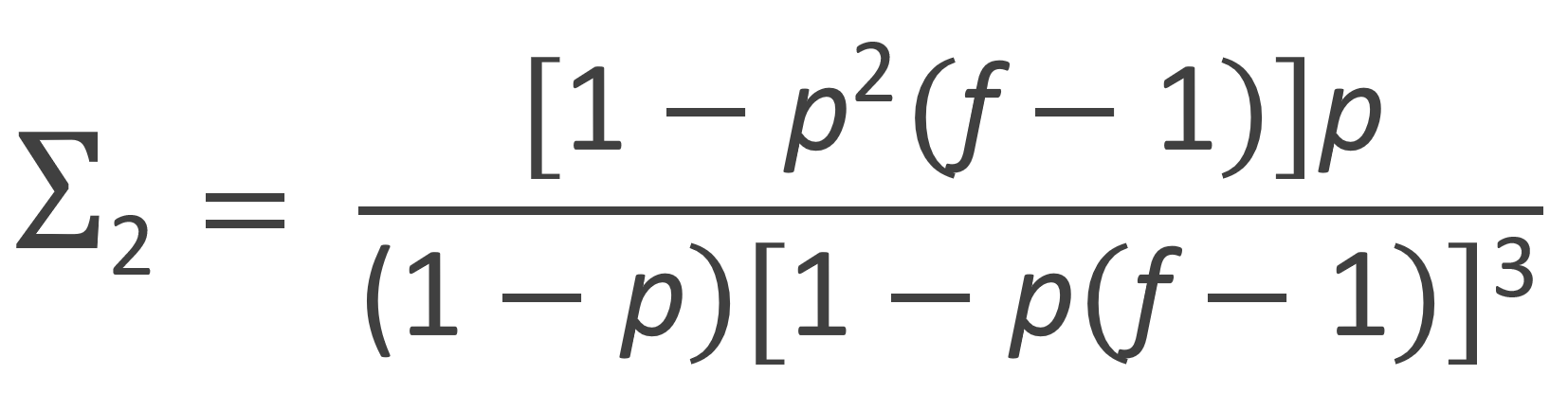 |
|
Number-Average Degree of Polymerization |
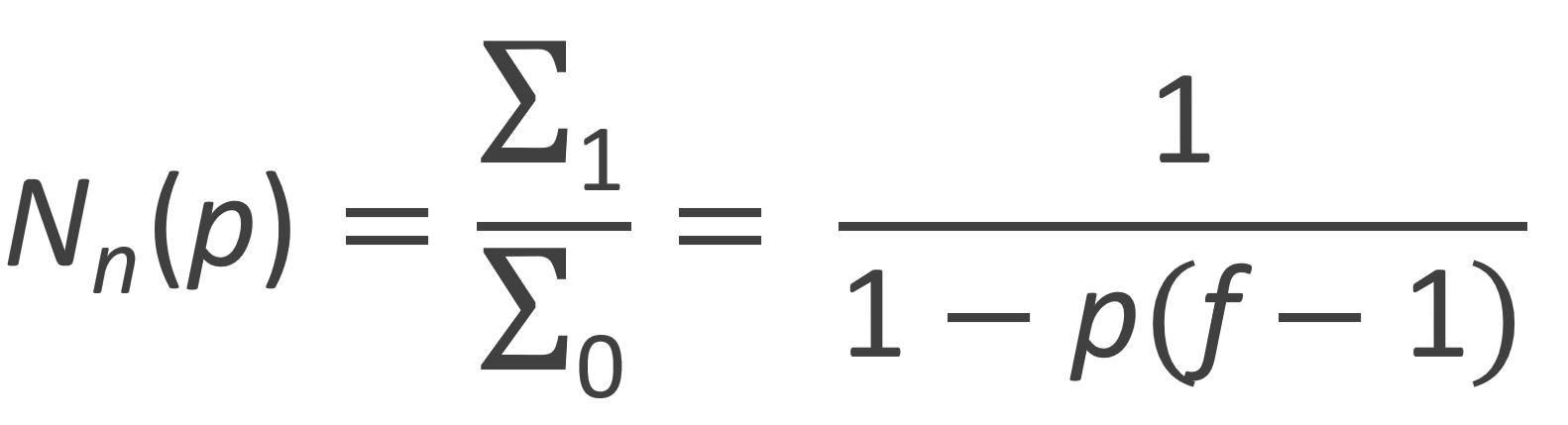 |
|
Weight-Average Degree of Polymerization |
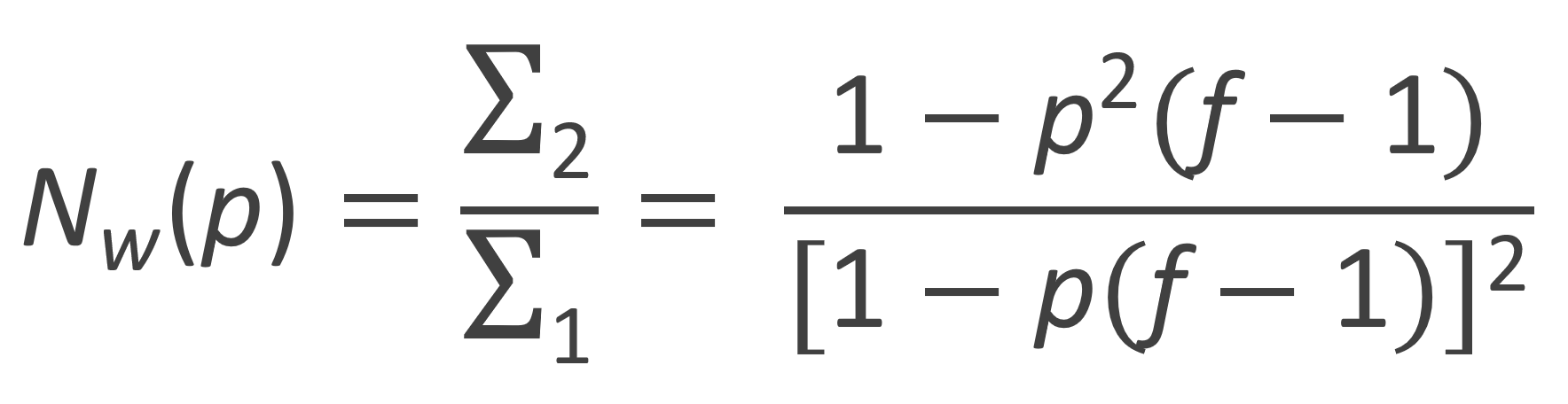 |
From these moments the number-average and weight-average degree of polymerization can be calculated:
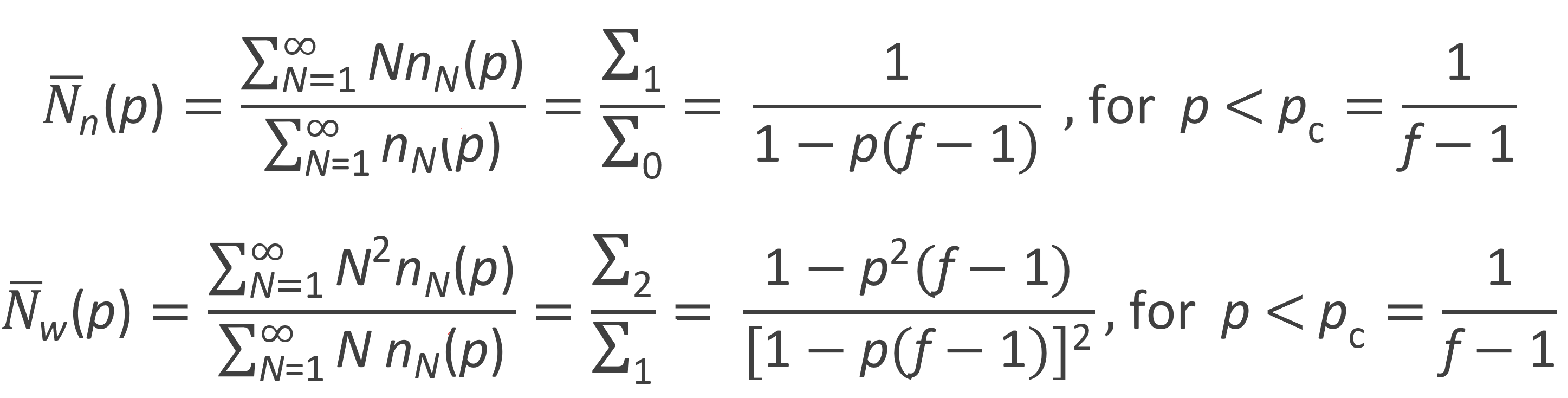
The weight fraction distribution of branched polymers derived from trifunctional monomers of the type AB2 is shown in the figure below for several extents of reaction p. The curves have been calculated with
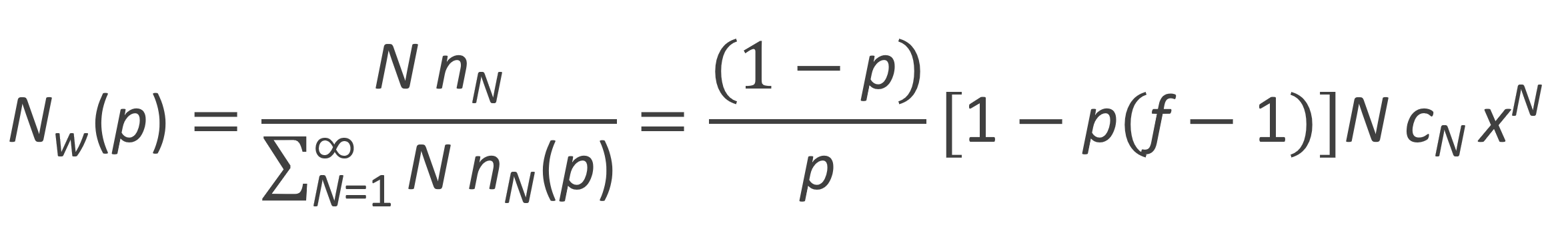
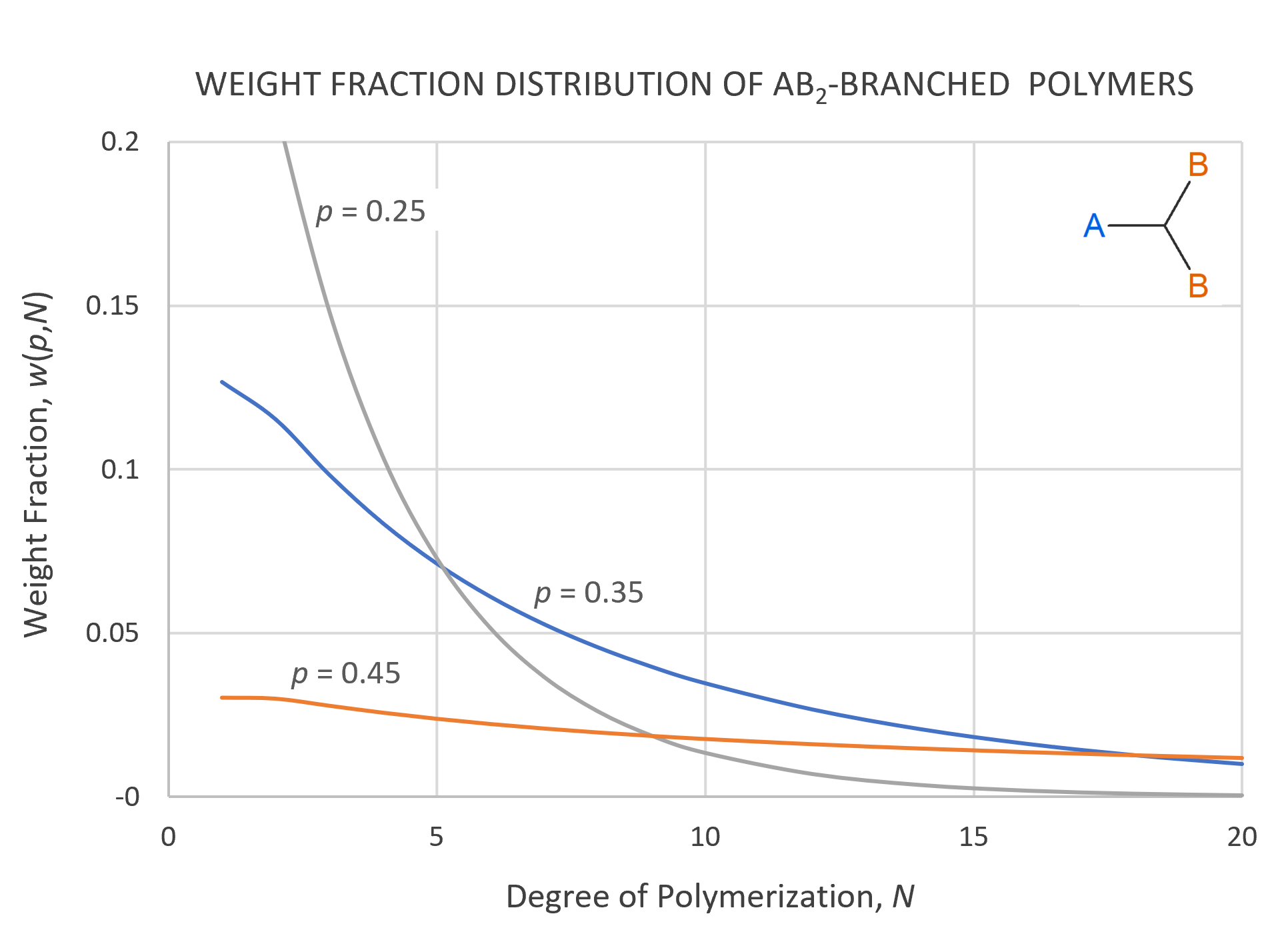
The curves show a broadening of the distribution with increasing extent of reaction and as p approaches pc = 0.5 (gel point) the curves merge with the x-axis. In other words, all weight fractions approach zero at p = pc because the monomers form one gigantic molecule. However, the area below the curve still remains equal to unity, which is only the case if the distribution becomes infinitely wide and infinitesimally high.
References and Further Readings
- H. Staudinger et al., Ber. Dtsch. Chem. Ges. 67, 1164 (1935) & 68, 232 (1935)
- P.J. Flory, J. Amer. Chem. Soc., 63, 3091 (1941)
- W. H. Stockmayer, J. Chem. Phys., 11, 45 (1943)
- P.J. Flory, J. Amer. Chem. Soc., 74, 2718 (1952)
- Paul L. Flory, Principles of Polymer Chemistry, Ithaca, New york, 1953
- Michael Rubinstein and Raph Colby, Polymer Physics, 1st Ed., New York 2003
- This assumption is called Flory's equal reactivity principle. It states that the probability pA that a given functional group A has reacted is equal to the ratio of reacted A's to total A's.