Polyepoxides (Epoxies)
Properties and Applications
Epoxies (also called polyepoxides) are an important class of reactive thermosetting
resins which contain at least one epoxide group. The resins can be reacted (cross-linked) either with themselves through catalytic homopolymerisation or with a wide range of co-reactants including polyfunctional amines, acid anhydrides, phenols, alcohols and thiols. The co-reactants
are often called hardener or curative, and the crosslinking reaction is commonly referred to as curing.
The thermosetting polyepoxides are known to have high temperature and chemical resistance.
When cured with a curative, the mechanical
and thermophysical properties depend on the type of curative and on the
epoxy to curative mix ratio.
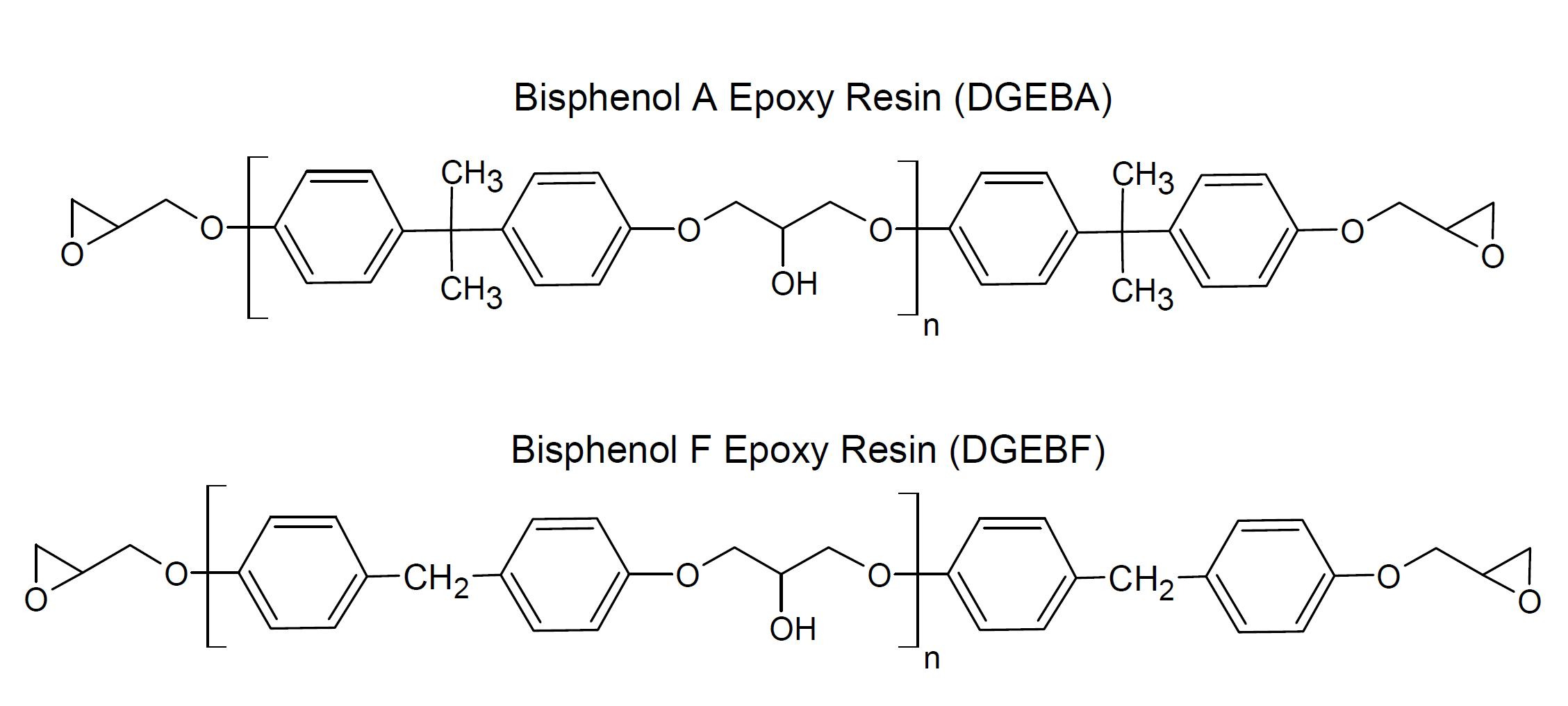
The most common and important epoxy compound is the diglycidyl ether of bisphenol A (DGEBA), which is formed from reacting epichlorhydrin with bisphenol A. The industrial grades normally contain some distribution of molecular weight, since pure DGEBA shows a strong tendency to form a crystalline solid upon storage at ambient temperature. Another very important epoxy resin is diglycidyl ether of bisphenol F (DGEBF). This resin is less viscous and yields thermosets of greater flexibility and toughness. On the downside, the resin is somewhat more expensive.
Epoxy resins are used across almost every industry. Important applications include metal coatings, potting compounds and encapsulations for electronics and electrical components, and thermal interface materials. They also find uses in fiber-reinforced plastics, coatings and structural adhesives. Due to their excellent heat and corrosion resistance and outstanding bond performance, they are widely used in the automotive and aerospace industry for demanding applications.