Polyacrylamides
Properties and Applications
Polyacylamides are high molecular weight water soluble or swellable polymers formed from acrylamide or its derivatives. The only commercially important polyacrylamide is poly(2-propenamide) which is simply called polyacrylamide or PAM. It is a non-ionic, watersoluble, and biocompatible polymer that can be tailored to meet a broad range of applications. The polymer can be synthesized as a simple linear chain or as a cross-linked structure. PAM increases the viscosity of water and encourages the flocculation of particles present in water. The cross-linked polymer can absorb and retain extremely large amounts of water because the amide groups form strong hydrogen bonds with water molecules.
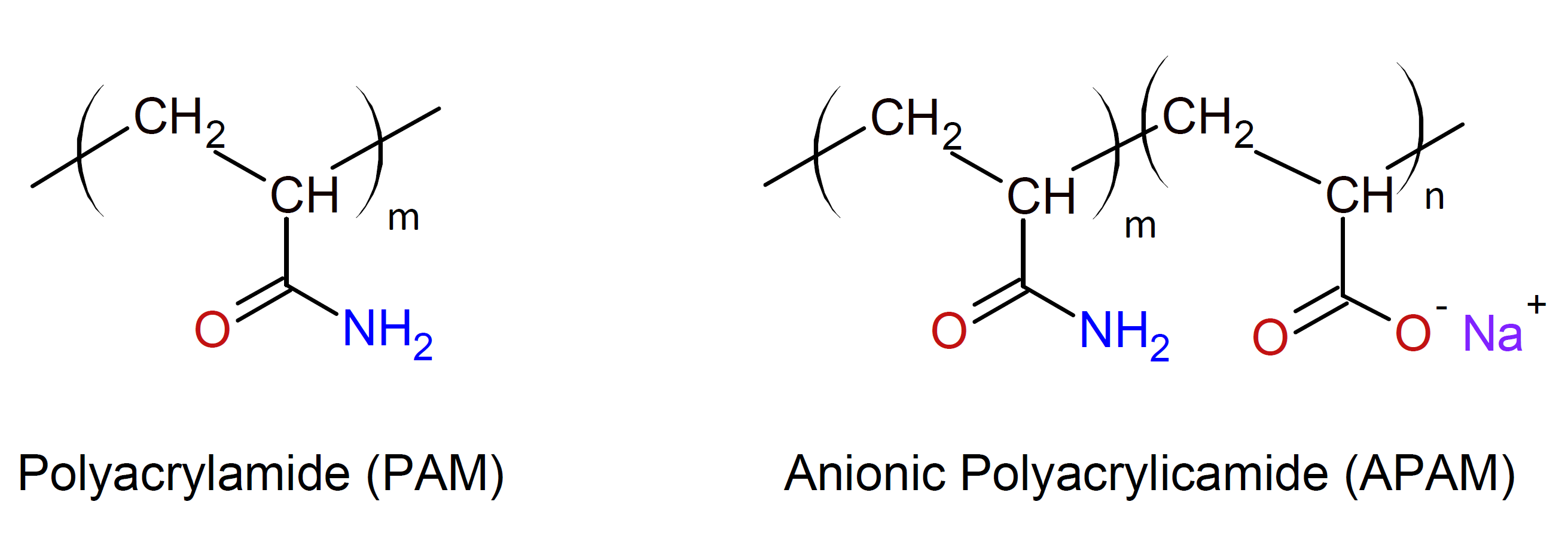
PAM and its sodium salt (APAM) are widely used as thickening agents, binders, super absorbents, soil conditioners, filtering aids, flocculating agents, crosslinkers, suspending agents, lubricants, and oil recovery agents. One of PAM's largest uses is waste water treatment. When added to waste water, it causes suspended particles to aggregate and to precipitate. PAM is also used to treat water from mineral mining operations. Another common use is oil extraction and recovery.
Some esters of PAM such as N,N-dimethylacrylamide (DMAA) and N-tert-butylacrylamide are also commercially available. These resins are often copolymerized with other monomers to improve tensile & impact strength of polyolefins, adherence to polar substrates, as well as dying, hygroscopic and antistatic properties of synthetic and semisynthetic fibers.1 DMAA is mainly used as a comonomer in plastic and formulated resin products including polyvinylic alcohol for fibers and papers treatment, acrylic adhesives, contact lenses, printing inks, cosmetics, and pharmaceuticals.1